Multiple choice question for engineering
Set 1
1. Which of the following is not a component of mass spectrometer?
a) Inlet system
b) Sweep generator
c) Ion transducer
d) Mass analyser
Answer
Answer: b [Reason:] Sweep generator is not a component of mass spectrometer. It is a component of NMR spectrometer.
2. Which of the following can be introduced into the ionization chamber directly?
a) Solid samples with low vapour pressure
b) Solid samples with high vapour pressure
c) Liquid samples with low density
d) Liquid samples with high density
Answer
Answer: a [Reason:] Solid samples with low vapour pressure can be introduced into the ionization chamber directly.
3. Inlet system is also known as which of the following?
a) Initial system
b) Sample reservoir
c) Sample handling system
d) Element injection system
Answer
Answer: c [Reason:] Inlet system introduces the sample into the ion source. Hence, it is called sample handling system.
4. Which of the following is normally done to convert the sample into gaseous state?
a) Sample is pressurized
b) Chemical reactions are made to occur
c) Sample is heated
d) Sample is cooled
Answer
Answer: c [Reason:] The sample must always be in gaseous state. Hence, liquid sample must be heated before introducing them into the ionization chamber.
5. Which of the following probes are used for the introduction of the sample?
a) Silica
b) Quartz
c) Graphite
d) Silver
Answer
Answer: a [Reason:] Solid samples with low vapour pressure are introduced into the entrance of the chamber. They are introduced using silica or platinum probe.
6. Which of the following is not a type of ionisation?
a) Field ionisation
b) Spontaneous ionisation
c) Spark ionisation
d) Chemical ionisation
Answer
Answer: b [Reason:] Spontaneous ionisation is not a type of ionisation. In mass spectrometer, ionisation is brought about by thermal or electrical energy.
7. Mass analyser is similar to which of the following in optical spectrometer?
a) Source
b) Monochromator
c) Detector
d) Sample
Answer
Answer: b [Reason:] Mass analyser is similar to monochromator in optical spectrometer. It separates ions according to their mass/charge ratio.
8. Which of the following is not one of the types of mass analyser?
a) Magnetic sector analyser
b) Frequency sweep analyser
c) Double focussing spectrometer
d) Time of flight analyser
Answer
Answer: b [Reason:] Frequency sweep analyser is not a type of mass analyser. There are many devices available for mass analysis.
9. Which of the following is not a type of ion detector used in mass spectrometers?
a) Electron multiplier
b) Flame emission detector
c) Faraday cup collector
d) Photographic plates
Answer
Answer: b [Reason:] Flame emission detector is not a type of ion detector used in mass spectrometers. Ion detectors produce current on the output side when there are ions on the input side.
10. Which of the following is used to inject liquid samples?
a) Hypodermic needle
b) Glass bulb
c) Capillary tube
d) Curvette
Answer
Answer: a [Reason:] Liquid samples are injected through hypodermic needles. It is vaporized at low pressure.
11. Under which of the following temperatures is the ionisation chamber maintained?
a) 100oC
b) 200oC
c) 300oC
d) 400oC
Answer
Answer: b [Reason:] The ionisation chamber is maintained at 200oC. It is also maintained at low pressure.
12. Given below is the block diagram of mass spectrometer. Identify the unmarked component.
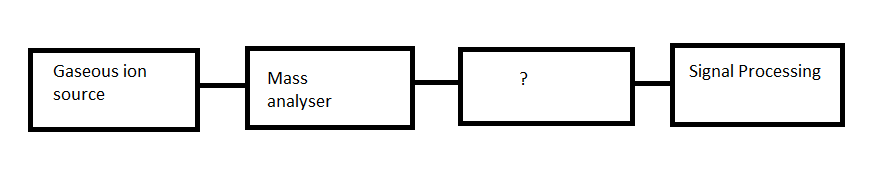
a) Inlet system
b) Ionisation chamber
c) Vacuum system
d) Ion transducer
Answer
Answer: d [Reason:] The unmarked component is ion transducer. It will give current at the output at its output side when ions are present on the input side.
13. Which of the following is not a characteristic of nebulizers that are commonly used?
a) Low cost
b) Low uptake rate
c) High efficiency
d) High uptake rate
Answer
Answer: d [Reason:] Commonly used nebulizers have low uptake rate. They also have low cost and high efficiency.
14. In glow discharge ion source, the sample is atomised by which of the following process?
a) Evaporation
b) Sputtering
c) Heating
d) Annealing
Answer
Answer: b [Reason:] In glow discharge ion source, the sample is atomised by the process of sputtering. It not only atomizes the sample but also provides means by which these atoms are ionized.
Set 2
1. Which of the following components are used to separate the nuclear spin energy states?
a) RF channels
b) Magnet
c) Sample probe
d) Sweep generator
Answer
Answer: b [Reason:] A magnet is used to separate the nuclear spin energy states. Permanent magnet or electromagnets can be used.
2. In frequency sweep method, which of the following parameters are varied continuously?
a) Magnetic field
b) RF signal
c) Sample concentration
d) Amplification factor
Answer
Answer: b [Reason:] In frequency sweep method, magnetic field is held constant. RF signal is swept or varied continuously.
3. In field sweep method, which of the following parameters are varied continuously?
a) Magnetic field
b) RF signal
c) Sample concentration
d) Amplification factor
Answer
Answer: a [Reason:] In frequency sweep method, RF signal is held constant. Magnetic field is swept or varied continuously.
4. Which of the following statements are not true about permanent magnets?
a) They are simple
b) They are inexpensive
c) They don’t require shielding
d) They don’t require power supply
Answer
Answer: c [Reason:] Permanent magnets are simple and inexpensive. But, permanent magnets require extensive shielding.
5. Which of the following is not true of electromagnets?
a) They are expensive
b) They require power supply
c) They don’t require cooling system
d) They don’t require extensive shielding
Answer
Answer: c [Reason:] Electromagnets require power supply. They also require cooling systems.
6. For high resolution work the magnetic field over the entire sample volume must be maintained uniform in space and time.
a) True
b) False
Answer
Answer: a [Reason:] For high resolution work the magnetic field over the entire sample volume must be maintained uniform in space and time. To do this all the components must be kept in specified conditions.
7. Which of the following must not be done to maintain the magnetic field over the sample uniform in space and time?
a) Large pole pieces need to be used
b) Pole faces must be polished
c) Wide pole gap must be present
d) Magnets can be permanent or electromagnet
Answer
Answer: c [Reason:] Narrow pole gap must be present to maintain the magnetic field over the sample uniform in space and time. The pole faces must be polished to optical tolerances.
8. The sample is contained in which of the following components?
a) Flask
b) Capillary tube
c) Curvette
d) Bore glass tube
Answer
Answer: d [Reason:] The sample is contained in a bore glass tube. It should be cylindrical and thin-walled.
9. The single coil probe supplies the RF radiation to the sample and also serves as a part of which of the following circuits?
a) RF channels
b) Magnet
c) Detector
d) Sweep generator
Answer
Answer: c [Reason:] The single coil probe supplies the RF radiation to the sample and also serves as a part of the detector circuit. It has only one coil.
10. Nuclear induction probes’ one coil is used for signal detection. What is the function of the other coil?
a) Sample irradiation
b) Magnet
c) Detector
d) Sweep generator
Answer
Answer: a [Reason:] Crossed coil probes are also called nuclear induction probes. One coil is used for irradiating the sample. The other coil is mounted orthogonally for signal detection.
11. Given below is the diagram of Cw NMR spectrometer. Identify the unmarked component.
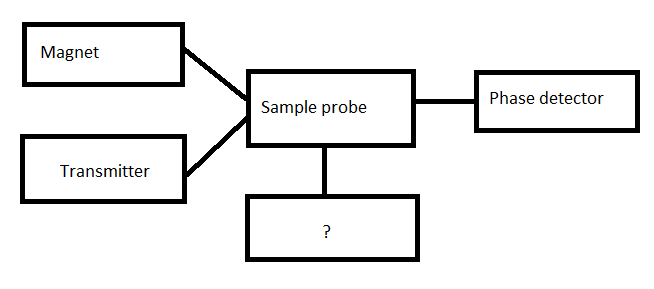
a) Recorder
b) RF channel
c) Receiver
d) Sweep generator
Answer
Answer: d [Reason:] The unmarked component is sweep generator. Either field or frequency can be swept.
12. The voltage generated by the receiver coil is small and it must be amplified.
a) True
b) False
Answer
Answer: a [Reason:] The voltage generated by the receiver coil is small and it must be amplified. It is them fed to the recorder or oscilloscope.
13. The amplification required for continuous-wave NMR is of the order of which of the following?
a) 101
b) 102
c) 103
d) 105
Answer
Answer: d [Reason:] The amplification required for continuous-wave NMR is of the order of 105. The spectrum can be recorded after amplification.
14. Which of the following can be used instead of magnets to produce the magnetic field?
a) Inductor
b) Motor
c) Generator
d) Superconducting solenoids
Answer
Answer: d [Reason:] Superconducting solenoids can be used instead of magnets to produce the magnetic field. The magnet must be stable and homogeneous.
15. How is the inhomogeneity of magnetic fields compensated?
a) With large magnetic fields
b) With small magnetic fields
c) By using two or more magnets
d) By providing required insulation
Answer
Answer: b [Reason:] The magnet must be stable and homogeneous. The inhomogeneity of magnetic fields is compensated with small magnetic fields.
Set 3
1. Which of the following gas permeable membrane is used for ammonia gas sensing electrode?
a) Silicon rubber
b) Microporous Teflon membrane
c) Fluorocarbon
d) Polythene
Answer
Answer: c [Reason:] Gas permeable membrane used for ammonia sensing electrode is fluorocarbon membrane. For each gas different membranes are used.
2. Which of the following gas permeable membrane is used for carbon dioxide gas sensing electrode?
a) Silicon rubber
b) PVC membrane
c) Fluorocarbon
d) Polythene
Answer
Answer: a [Reason:] Gas permeable membrane used for carbon dioxide sensing electrode is silicon rubber membrane. For each gas different membranes are used.
3. The response time of biocatalytic membrane electrode is poor when used with complex organic molecules.
a) True
b) False
Answer
Answer: b [Reason:] The response time of biocatalytic membrane electrode is good even when used with complex organic molecules. But, the membrane is costly.
4. Which of the following reference electrode is placed in the solution of carbon dioxide electrode?
a) Calomel electrode
b) Silver/silver chloride electrode
c) Mercury/mercury sulphate electrode
d) Glass electrode
Answer
Answer: b [Reason:] A silver/silver chloride reference electrode is placed in the solution. A glass electrode is also placed in the solution but it is not a reference electrode.
5. Biocatalytic membrane electrode cannot be used for the measurement of which of the following gases?
a) Ammonia
b) Carbon dioxide
c) Hydrogen
d) Nitrogen
Answer
Answer: d [Reason:] Biocatalytic membranes cannot be used for the measurement of nitrogen. It can be used for the measurement of other three gases.
6. The biocatalytic membrane used in ammonia selective electrode is which of the following?
a) Urea
b) Urease
c) Acrylamide
d) Polyacrylamide
Answer
Answer: b [Reason:] The biocatalytic membrane used in ammonia selective electrode is urease. It is used for the detection of ammonia.
7. Which of the following materials are used as gel to which biocatalytic membrane is fixed in ammonia selective electrode?
a) Urea
b) Urease
c) Acrylamide
d) Polyacrylamide
Answer
Answer: c [Reason:] The material used as gel to which biocatalytic membrane is fixed in ammonia selective electrode is acrylamide. This is attached to the ion selective electrode directly.
8. The biocatalytic membrane is attached to the glass electrode using which of the following materials?
a) Nylon mesh
b) Teflon
c) Silicon rubber
d) Polythene
Answer
Answer: a [Reason:] The biocatalytic membrane is attached to the glass electrode using nylon mesh. Cellophane film can also be used.
9. The response time of the biocatalytic electrode is a function of thickness of the enzyme layer.
a) True
b) False
Answer
Answer: a [Reason:] The response time of the biocatalytic electrode is a function of thickness of the enzyme layer. It is also a function of diffusion of the layer in the sample solution.
10. In carbon dioxide electrode, the membrane separates which of the following?
a) Sodium carbonate, magnesium chloride
b) Magnesium hydrogen carbonate, sodium chloride
c) Sodium hydrogen carbonate, sodium chloride
d) Magnesium carbonate, magnesium chloride
Answer
Answer: b [Reason:] In carbon dioxide electrode, the membrane separates sodium hydrogen carbonate and sodium chloride. Sodium hydrogen carbonate is the internal electrolyte.
Set 4
1. Which of the following is not a type of optics employed in electron probe microanalyser?
a) Electron optics
b) Light optics
c) X-ray optics
d) Gamma optics
Answer
Answer: d [Reason:] Gamma optics is not a type of optics used in electron probe microanalyser. Electron optics, light optics and X-ray optics are employed.
2. The electron optics consists of an electron gun followed by which of the following components?
a) Collimator
b) Slit
c) Amplifier
d) Electron beam probe
Answer
Answer: d [Reason:] The electron optics consists of an electron gun followed by electron beam probe. This is formed by two electro-magnetic lenses.
3. The specimen is mounted inside which of the following components?
a) Test tube
b) Glass capillary tube
c) Vacuum column
d) Curvette
Answer
Answer: c [Reason:] The specimen is mounted inside the vacuum column in the instrument. It is under the beam as the target.
4. The electrons are accelerated by voltages in which of the following ranges?
a) 5 and 50kV
b) 50 and 500kV
c) 500 and 5000kV
d) 25 and 250kV
Answer
Answer: a [Reason:] The whole system operates in a vacuum. The electrons are accelerated by voltages in the range of 5 and 50kV.
5. Electron probe microanalyser is a method of destructive elemental analysis.
a) True
b) False
Answer
Answer: a [Reason:] Electron probe microanalyser uses a finely focussed electron beam to excite the X-rays. It is a method of destructive elemental analysis.
6. Which of the following is the effective resolution limit in electron probe microanalyser?
a) 1mm
b) 10mm
c) 100mm
d) 1000mm
Answer
Answer: a [Reason:] The electrons spread laterally and longitudinally in the sample by approximately 1mm. Hence, the effective resolution limit is 1mm.
7. Micro probe analyser cannot be used on inhomogeneous material.
a) True
b) False
Answer
Answer: b [Reason:] Micro probe analyser can be used on inhomogeneous material. It can also be focussed on
a very small area.
8. X-ray emission must be analysed against a background of _______ radiation.
a) Blue
b) Yellow
c) White
d) Green
Answer
Answer: c [Reason:] X-ray emission must be analysed against a background of white radiation. Microprobe has poorer sensitivity than XRF spectrometer.
9. Which of the following is the limit of detectability of electron microprobe analyser?
a) 10-14 g
b) 10-140 g
c) 10-7 g
d) 10-70 g
Answer
Answer: a [Reason:] Electron microprobe analyser allows the analysis of extremely small objects. The limit of detectability is 10-14 g.
10. The alternative method using laser does not analyse vapours by which of the following methods?
a) Mass spectrometer
b) Optical emission
c) Absorption photometry
d) X-ray photometry
Answer
Answer: d [Reason:] The alternative method using laser does not analyse vapours by X-ray photometry. This method is gaining popularity.
Set 5
1. The kinetic energy of the photoelectron energies is dependent on _________ of the atom, which makes XPS useful to identify the oxide state.
a) Mass
b) Charge
c) Chemical environment
d) Volume
Answer
Answer: c [Reason:] The kinetic energy of the photoelectron energies is dependent on chemical environment of the atom, which makes XPS useful to identify the oxide state. It also helps to identify the ligands of the atom.
2. Ion etching techniques provides the depth profiling from the surface.
a) True
b) False
Answer
Answer: a [Reason:] Ion etching techniques provides the depth profiling from the surface. Binding energy can also be used.
3. Electron spectroscopy is based on the ionization phenomenon.
a) True
b) False
Answer
Answer: a [Reason:] Electron spectroscopy is based on the ionization phenomenon. It can be ionization of photon or electron.
4. The kinetic energy of the ejected photoelectron is dependent upon the energy of which of the following?
a) Ions around
b) Photons around
c) Material
d) Impinging photon
Answer
Answer: d [Reason:] The kinetic energy of the ejected photoelectron is dependent upon the energy of impinging photon. A free electron is ejected.
5. ESCA gives sufficient chemical information up to a depth about ________ armstrong in metals.
a) 5-20
b) 15-40
c) 40-100
d) 100-200
Answer
Answer: a [Reason:] ESCA gives sufficient chemical information up to a depth about 5-20 armstrong in metals. ESCA is also known as X-ray photoelectron spectroscopy.
6. ESCA gives sufficient chemical information up to a depth about ________ armstrong in polymers.
a) 5-20
b) 15-40
c) 40-100
d) 100-200
Answer
Answer: c [Reason:] ESCA gives sufficient chemical information up to a depth about 40-100
armstrong in polymers. ESCA is also known as X-ray photoelectron spectroscopy.
7. ESCA gives sufficient chemical information up to a depth about ________ armstrong in oxide.
a) 5-20
b) 15-40
c) 40-100
d) 100-200
Answer
Answer: b [Reason:] ESCA gives sufficient chemical information up to a depth about 15-40 armstrong in oxide. ESCA is also known as X-ray photoelectron spectroscopy.
8. ESCA can identify elements in the periodic table above which of the following?
a) Carbon
b) Boron
c) Helium
d) Potassium
Answer
Answer: c [Reason:] ESCA can identify elements in the periodic table above helium. Adjacent elements are clearly distinguished.
9. Discrete electrons cannot be observed in electron ionization of an atom due to which of the following reasons?
a) Environmental disturbances
b) Same mass
c) Same charge
d) Electron- electron interaction
Answer
Answer: d [Reason:] Discrete electrons cannot be observed in electron ionization of an atom because of electron-electron interaction. Therefore, ESCA cannot be observed when using electron ionization.
10. ESCA focusses on which of the following information?
a) Mass of the electron
b) Charge of the electron
c) Binding energy of the electron
d) Mass of atoms
Answer
Answer: c [Reason:] ESCA focusses on binding energy of the electrons. It focusses on the binding energy which the electrons had before they left the atom.
11. In the spectrum, two main peaks at _________ and ________ are observed.
a) 284.6, 532.5
b) 248.6, 523.5
c) 264.8, 535.2
d) 246.8, 553.2
Answer
Answer: a [Reason:] In the spectrum, two main peaks at 284.6 and 532.5 are observed. The unit for counting energy is electron-volt.
12. 284.6 eV matches which of the following specific atom type?
a) Carbon
b) Oxygen
c) Nitrogen
d) Argon
Answer
Answer: a [Reason:] Each energy matches a specific atom type. 284.6 eV matches carbon.
13. 532.5 eV matches which of the following specific atom type?
a) Carbon
b) Oxygen
c) Nitrogen
d) Argon
Answer
Answer: b [Reason:] Each energy matches a specific atom type. 532.5 eV matches carbon.
14. By studying which of the following can we determine if the surface corresponds to C-O or C=O chemical form?
a) Mass of the electron
b) Energy of the carbon peak
c) Binding energy
d) Charge of electron
Answer
Answer: b [Reason:] By studying energy of the carbon peak it can be determined if the surface corresponds to C-O or C=O chemical form. Thus, the specimen chemical composition can be obtained.
15. Which of the following is the detection limit of ESCA?
a) 0.1% monolayer
b) 0.5% monolayer
c) 1% monolayer
d) 2% monolayer
Answer
Answer: a [Reason:] The detection limit of ESCA is 0.1% monolayer. It has no x-y resolution.
